A bi-metallic MOF catalyst via sensitive detection & adsorption of Fe3+ ions for size-selective reaction prompting†
Abstract
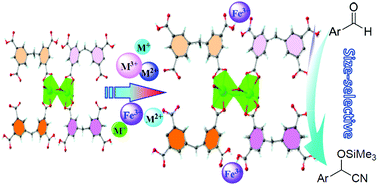
A cadmium(II)-based MOF, Cd-MDIP, was successfully prepared by hydrothermal reaction between the tetra-carboxylic ligand 5,5′-methylenebisophthalic acid (H4MDIP) and cadmium perchlorate. The X-ray crystal structure analysis showed that there are two uncoordinated carboxyl groups in each ligand and a 1D elliptical channel along the [001] direction. Because of the existence of uncoordinated carboxyl groups within open frameworks, Cd-MDIP exhibited a high sensitivity (Stern–Volmer constant KSV = 4.13 × 104 L mol−1) and a low detection limit (80 nM) for Fe3+ ions in pure water, which is much lower than the national standard for Fe3+ in daily drinking water (5.4 μM) set by the Ministry of Environmental Protection of P. R. China. Most importantly, Cd-MDIP also featured the ultrahigh adsorption of Fe3+ in aqueous solution that cannot be destroyed even by EDTA/base. Importantly, the MOF material (Cd-MDIP⊃Fe3+) after adsorbing Fe3+ could act as the first example of an excellent bi-metallic Lewis-acid catalyst for the cyanosilylation reaction of aromatic aldehydes in a size-selective fashion, and its efficiency was almost 10-times higher than that of the original Cd-MDIP.


 Please wait while we load your content...
Please wait while we load your content...