Mechanism of H adatoms improving the O2 reduction reaction on the Zn-modified anatase TiO2 (101) surface studied by first principles calculation†
Abstract
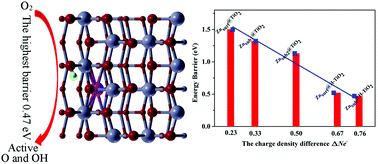
First principles calculations were performed to cast insight into the mechanism of the improvement of O2 reduction reaction (ORR) activity by Zn and H interstitials on the anatase TiO2 (101) surface. For the Zn-modified anatase TiO2 (101) surface, both surface and subsurface Zn interstitials could contribute to O2 adsorption and dissociation, but the dissociation barriers of O2 molecules are still too high, which limits the ORR activity. After a H adatom is introduced onto the Zn-modified anatase TiO2 (101) surface, the highest energy barriers are greatly reduced compared with those of the Zn-modified surface. Meanwhile, it is observed that the dissociation barriers decrease almost linearly with the increase of the charge difference of adsorption O2 between initial and transition state configurations. Specifically, subsurface Zn and surface H interstitials facilitate O2 dissociation and subsequent oxidation reactions, and further frequency analysis shows that these dissociation processes are frequent even at the room temperature of 300 K. In a word, this work provides a theoretical support to design a high ORR activity catalyst of the TiO2 nanocrystal comparable to precious Pt catalysts.


 Please wait while we load your content...
Please wait while we load your content...