Carbon–sulfur bond formation by reductive elimination of gold(iii) thiolates†
Abstract
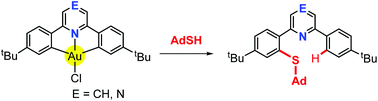
Whereas the reaction of the gold(III) pincer complex (C^N^C)AuCl with 1-adamantyl thiol (AdSH) in the presence of base affords (C^N^C)AuSAd, the same reaction in the absence of base leads to formation of aryl thioethers as the products of reductive elimination of the Au–C and Au–S ligands (C^N^C = dianion of 2-6-diphenylpyridine or 2-6-diphenylpyrazine). Although high chemical stability is usually taken as a characteristic of pincer complexes, results show that thiols are capable of cleaving one of the pincer Au–C bonds. This reaction is not simply a function of S–H acidity, since no cleavage takes place with other more acidic X–H compounds, such as carbazole, amides, phenols and malonates. The reductive C–S elimination follows a second-order rate law, −d[1a]/dt = k[1a][AdSH]. Reductive elimination is enabled by displacement of the N-donor by thiol; this provides the conformational flexibility necessary for C–S bond formation to occur. Alternatively, reductive C–S bond formation can be induced by reaction of pre-formed thiolates (C^N^C)AuSR with a strong Brønsted acid, followed by addition of SMe2 as base. On the other hand, treatment of (C^N^C)AuR (R = Me, aryl, alkynyl) with thiols under similar conditions leads to selective C–C rather than C–S bond formation. The reaction of (C^N^C)AuSAd with H+ in the absence of a donor ligand affords the thiolato-bridged complex [{(C^N–CH)Au(μ-SAd)}2]2+ which was crystallographically characterised.


 Please wait while we load your content...
Please wait while we load your content...