Influence of the catalyst structure in the cycloaddition of isocyanates to oxiranes promoted by tetraarylstibonium cations†
Abstract
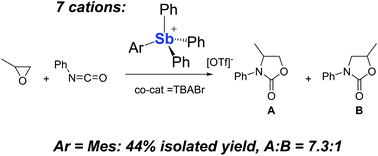
In the context of our work on electron deficient group 15 cations as Lewis acid catalysts, we have synthesized the triflate salts of a series of tetraarylstibonium cations of general formula [ArSbPh3]+ with Ar = Mes (4+), o-(dimethylamino)phenyl (5+), and o-((dimethylamino)methyl)phenyl (6+). These new cationic antimony derivatives, along with the known [Ph4Sb]+ (1+), 1-naphthyltriphenylstibonium (2+), and [(Ant)SbPh3]+ (3+), have been evaluated as catalysts for the cycloaddition of oxiranes and isocyanates under mild conditions. While all stibonium cations favor the 3,4-oxazolidinone products, the reactivities of 5+ and 6+ are hindered by the ancillary amino donor which quenches the Lewis acidity of the antimony center. A comparison of the other stibonium cations shows that 4+ is the most selective catalyst.
- This article is part of the themed collection: Frontiers in coordination chemistry and its applications


 Please wait while we load your content...
Please wait while we load your content...