Formation and reactions of active five-membered phosphane/borane frustrated Lewis pair ring systems†
Abstract
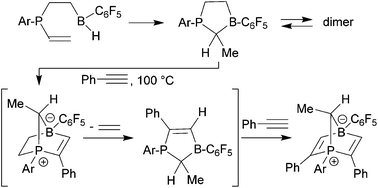
The reactive five-membered frustrated P/B Lewis pair 6a was generated from Tipp-P(vinyl)2 (Tipp: 2,4,6-triisopropylphenyl) by a series of anti-Markovnikov and Markovnikov hydroboration reactions. The in situ generated compound dimerized to give 7a under kinetic control and the dimer pair 8a/9a under thermodynamic control. The Mes*-P(vinyl)2 analogue (Mes*: 2,4,6-tri-tert-butylphenyl) reacts in a similar way, but in this case the thermodynamic dimers are observed at r.t. whereas the monomer 6b is the dominant species at 80 °C in solution. It is an active dihydrogen splitting reagent. The in situ generated monomeric five-membered P/B FLPs undergo addition reactions to various organic π-reagents. With benzaldehyde or phenylacetylene this gave the respective zwitterionic heteronorbornene or -norbornadiene type products, the latter with liberation of ethylene.


 Please wait while we load your content...
Please wait while we load your content...