Decaborane anion tautomerism: ion pairing and proton transfer control†
Abstract
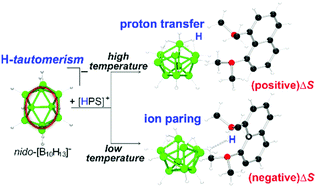
The reaction of 1,8-bis(dimethylamino)naphthalene—which is often referred by the trade name Proton-Sponge (PS)—with decaborane in hexane afforded [HPS][B10H13] (1) salt as a pale-yellow precipitate. Variable-temperature NMR studies allowed the full assignment of 1H and 11B spectra for this familiar ten-vertex polyhedral anion. In addition, this work reveals that an increase in the temperature leads to the intramolecular exchange of three B–H–B hydrogen atoms around the hexagonal face of the boat-shaped cluster. This previously unrecognised H-tautomerism complements the long-known low-energy proton exchange of only one of the bridging hydrogen atoms. The temperature dependent proton fluxional behaviour controls the molecular environment of the polyhedral cage, averaging the negative charge of the anion. The result is a debilitation of the cation–anion interactions in solution, favouring the transfer of the proton from the organic aromatic cation, [HPS]+, to the polyhedral anion, [B10H13]−. This proton transfer affords Proton-Sponge and decaborane, increasing the entropy of the system and sustaining an equilibrium which at high temperatures shifts toward the neutral reactants and at low temperatures moves toward the ionic products. A single X-ray diffraction analysis of 1 is discussed.


 Please wait while we load your content...
Please wait while we load your content...