Reversible modulation of the redox characteristics of acid-sensitive molybdenum and tungsten scorpionate complexes†
Abstract
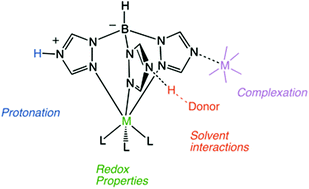
The large-scale synthesis of the scorpionate ligand Ttz (hydrotris(1,2,4-triazol-1-yl)borate) is reported as well as syntheses of Group VI complexes K[M(L)(CO)3] and M(L)(NO)(CO)2 (L = Ttz or Tp (hydrotris(pyrazol-1-yl)borate), M = Mo or W). The redox characteristics of the metal in these Ttz complexes are shown to be reversibly modulated by interactions between the exo-4-N lone pairs of the triazolyl rings and Brønsted or Lewis acids. The basicity of the scorpionate ligand in [M(Ttz)(CO)3]− is quantified (pKaH2O values range from 1.1 to 4.6) and found to be dependent on both the oxidation state and identity of the metal. In the presence of Brønsted acids, the observed redox behavior for the one-electron oxidation of the Group VI metal center is consistent with a proton-coupled electron transfer (PCET). Indeed, for both Mo and W derivatives, a one-electron oxidation decreases the pKa by ∼3.5 units.


 Please wait while we load your content...
Please wait while we load your content...
