A ternary Fe(ii)-terpyridyl complex-based single platform for reversible multiple-ion recognition†
Abstract
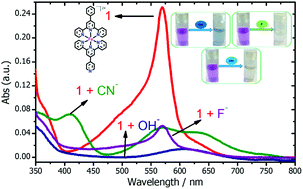
Multiple ion-recognition activity by a ternary Fe(II)-terpyridyl complex, [Fe(PhT)(PT)]2+ (1) (PhT = 4′-phenyl-2,2′:6′,2′′-terpyridine; PT = 4′-pyridyl-2,2′:6′,2′′-terpyridine), is demonstrated for cyanide (CN−), fluoride (F−) and hydroxide (OH−) ions in an aqueous medium with sufficient sensitivity, fast response, reproducibility and selectivity with a dual optical read-out. The sensing event was reversible with the “by-eye” visualization of back and forth colour changes. Three cyanide ions replaced PT from 1, as observed from the crystal structure of the 1 + CN− couple. Fluoride and hydroxide ions appeared to show multivariate interactions with 1. Observed structural and spectral changes correlated well with theoretical calculations. A string of cations at quantitative levels (Ag+/Hg2+/Fe2+/Fe3+) was used to decouple the 1 + anion complex to yield 1, which enabled the recognition of these cations while permitting the reuse of 1 for at least five set–reset cycles.


 Please wait while we load your content...
Please wait while we load your content...