A substituent-tolerant synthetic approach to N/P-“loaded” heteroarenes†
Abstract
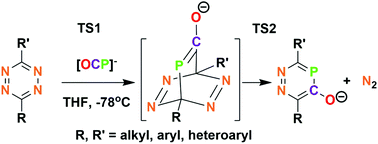
Tetrazines react with OCP−1 through a reverse electron demand Diels–Alder process to produce 3,6-disubstituted-1,2,4-diazaphosphinin-5-olates. DFT calculations reveal that both Diels–Alder and subsequent aromatization barriers are low for both EWG and ED tetrazine substituents. The structure of the solid sodium salt shows the interaction of Na+ with aryloxide and also both nitrogens of a neighboring anion, leading to coordination polymer character. 1,2,4-Diazaphosphinin-5-olates react as nucleophiles towards MeI and R3SiCl, respectively, and were installed on the (Ph3P)2Ru(CO)H fragment to investigate their properties as ligands.


 Please wait while we load your content...
Please wait while we load your content...
