Metal complexes of a novel heterocyclic benzimidazole ligand formed by rearrangement-cyclization of the corresponding Schiff base. Electrosynthesis, structural characterization and antimicrobial activity†
Abstract
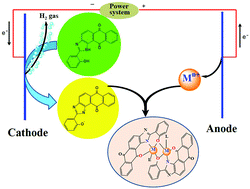
The electrochemical oxidation of anodic metals (M = cobalt, nickel, copper, zinc and cadmium) in a solution of the ligand 1H-anthra[1,2-d]imidazol-6,11-dione-2-[2-hydroxyphenyl] [H2L] afforded homoleptic [ML] compounds. The addition to the electrochemical cell of coligands (L′) such as 2,2′-bipyridine (bpy) or 1,10-phenanthroline (phen) allowed the synthesis, in one step, of heteroleptic [MLL′] compounds. The crystal structures of H2L (1), [CoL(MeOH)]2 (2), [CoL(phen)]2 (3), [NiL(bpy)]2 (4), [CuL(bpy)] (5), [CuL(phen)] (6) and [CdL(bpy)]2 (7) have been determined by X-ray diffraction techniques. The crystal structures of 2, 3, 4 and 7 consist of dimeric species in which both metallic atoms are connected through two phenolate bridges in a penta-coordinated (2) or hexa-coordinated (3, 4 and 7) environment. Copper compounds 5 and 6 are monomeric species with the metal in a pentacoordinated [N4O] environment. In all the compounds, the main interactions responsible for the crystal packing are classic (N–H⋯O, O–H⋯N and O–H⋯O) and non-classic (C–H⋯O and C–H⋯N) hydrogen bond interactions, and π interactions (π–π-stacking and C–H⋯π). All compounds were also characterized by microanalysis, IR spectroscopy, FAB mass spectrometry and 1H NMR spectroscopy. Magnetic susceptibility data were measured for 2–4 over the temperature range 2–300 K, and their analysis has revealed the occurrence of intramolecular antiferromagnetic coupling for 2 (J = −2 cm−1) and ferromagnetic coupling for 3 (J = 7.8 cm−1) and 4 (J = 2.8 cm−1) [J being the isotropic magnetic coupling parameter]. The nature of the magnetic coupling in 2–4 is correlated with the magnitude of the M–Ophenolate–M angle between the phenolate bridge and the metallic centers [M(II) = Co, Ni]. The in vitro antimicrobial properties of the novel ligand and its metal complexes were detected against Gram positive and Gram negative bacteria and fungi. [NiL(bpy)]2 and all tested Cd(II) complexes were the most active compounds, showing the highest inhibitory effect against bacilli (MIC 1.5–3 μg mL−1) and Sarcina, Streptococci and Haemophilus influenzae bacterial strains (MIC 12–50 μg mL−1), while almost no antifungal properties were observed.


 Please wait while we load your content...
Please wait while we load your content...