N- and S-donor leaving groups in triazole-based ruthena(ii)cycles: potent anticancer activity, selective activation, and mode of action studies†
Abstract
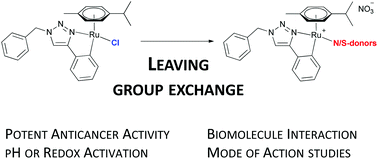
A series of 11 novel ruthenium(II) arene complexes [Ru(p-cym)(trzC^N)L]NO3 based on the cycloruthenated 1,2,3-triazole scaffold (trzC^N) bearing different N- or S-donor leaving groups (L) were prepared. These complexes exhibited strongly diverging pH-dependent stability profiles, but consistently exerted antiproliferative effects in the low micromolar range in three cancer cell lines (A549, SW480, CH1/PA-1). The interaction with biomolecules was correlated to dissociation of the monodentate leaving group. Under oxidative conditions, the stably bound dimethylsulfide ligand (3a) undergoes oxidation, while metal coordination is maintained, affording the labile DMSO complex (3b). Rationalization of the homogenous antiproliferative activities was attempted by determination of the cellular accumulation and lipophilicity indices (ϕ0). Investigations on their mechanism of action revealed that these metalacycles are inducers of apoptosis, exert a slight antioxidative effect in cell culture studies, but have no DNA intercalatory activity.


 Please wait while we load your content...
Please wait while we load your content...