Stoichiometrically controlled chirality inversion in zinc bisporphyrinate–monoamine complexes†
Abstract
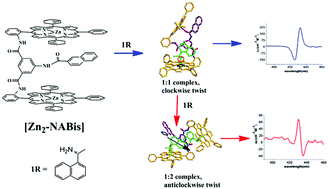
We have designed and synthesized a novel amide-linked bisporphyrin with a 2-naphthalenecarboxamide substituent on the linker. The chirality sensing ability of this zinc porphyrinate towards chiral monoamines has been examined. Circular dichroism studies indicate chirality transfer from chiral monoamines to the corresponding host–guest complexes. Interestingly, when optically pure 1-(1-naphthyl)ethylamine is used as a guest, stoichiometrically controlled chirality inversion occurs in the formation of the corresponding zinc bisporphyrinate–monoamine complexes. Both CD and NMR spectra indicate that 1 : 1 and 1 : 2 complexes are formed in solution. DFT calculations suggest that steric interactions between the substituent of the linker and the guest in the 1 : 2 complex are responsible for this unprecedented observation. Our studies provide a unique bisporphyrin system for demonstrating that chirality transfer to the host can be tuned by changing the substituent of the linker.
- This article is part of the themed collection: Dalton Transactions Inorganic Symposia


 Please wait while we load your content...
Please wait while we load your content...