Water molecule induced reversible single-crystal-to-single-crystal transformation between two trinuclear Fe(ii) complexes with different spin crossover behaviour†
Abstract
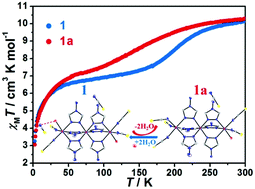
A 4-amino-1,2,4-triazole (NH2-trz) based linear trinuclear Fe(II) complex of [Fe3(NH2-trz)6(SCN)4(H2O)2](SCN)2·H2O (1) has been hydrothermally synthesized by the reaction of a NH2-trz ligand with FeSO4. A single crystal of complex 1 is able to extrude one lattice water and one coordinated water, leading to another single crystal of complex 1a with a formula [Fe3(NH2-trz)6(SCN)5(H2O)](SCN) by heating. Complex 1a can be reversed to 1 upon the reabsorption of water. Single-crystal X-ray diffraction analysis reveals that these two complexes consist of a linear trinuclear core where a central Fe1 ion is coordinated to six nitrogen atoms from six bridged NH2-trz ligands and one of the terminal Fe2 ions is coordinated to five nitrogen atoms and one oxygen atom. The terminal Fe3 ion in 1 coordinates to five nitrogen atoms and one oxygen atom; however, the terminal Fe3 ion in 1a coordinates to six nitrogen atoms. Magnetic susceptibility measurements demonstrate that they exhibited gradual spin crossover (SCO) for the central Fe(II) ion; however, different spin transition temperatures were displayed for complexes 1 and 1a (T1/2 = 202 K for 1 and 160 K for 1a). These different spin transition temperatures arise from the different intermolecular hydrogen bonding interactions in the two complexes.
- This article is part of the themed collection: Dalton Transactions Inorganic Symposia


 Please wait while we load your content...
Please wait while we load your content...