Neutral ligand TIPA-based two 2D metal–organic frameworks: ultrahigh selectivity of C2H2/CH4 and efficient sensing and sorption of Cr(vi )†
Abstract
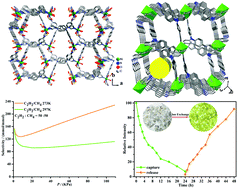
One neutral tripodal semi-rigidity ligand tri(4-imidazolylphenyl)amine (TIPA) with excellent hole-transfer nature, was selected as a linker to construct MOFs. Two two-dimensional (2D) microporous metal–organic frameworks (MOFs) were synthesized solvothermally: [Ni(TIPA)(COO−)2(H2O)]·2(DMF)2(H2O) (1) and [Cd(TIPA)2(ClO4−)2]·(DMF)3(H2O) (2). Compound 1 incorporated carboxylic groups into the channel and exhibited the high capacity of light hydrocarbons as well as the remarkable selectivity of C2H2/CH4. The value is in excess of 100 at room temperature, which is the highest value reported to date. Compound 2, as a cationic framework with high water stability, was not only applied as a sensor, displaying the ultrahigh sensitivity against Cr2O72− with a detection limit as low as 8 ppb, but also possessed excellent Cr(VI) sorption with good repeatability in aqueous solution. This study provides an efficient strategy to design cationic MOFs for the selective separation of light hydrocarbons and the sensing and trapping of toxic chromate for the purification of water.
- This article is part of the themed collection: Spotlight Collection: 2D Materials Chemistry


 Please wait while we load your content...
Please wait while we load your content...