Quadratic and cubic hyperpolarizabilities of nitro-phenyl/-naphthalenyl/-anthracenyl alkynyl complexes†‡
Abstract
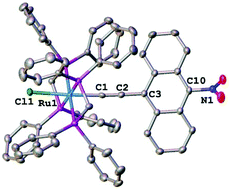
1-Nitronaphthalenyl-4-alkynyl and 9-nitroanthracenyl-10-alkynyl complexes [M](C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-4-C10H6-1-NO2) ([M] = trans-[RuCl(dppe)2] (6b), trans-[RuCl(dppm)2] (7b), Ru(PPh3)2(η5-C5H5) (8b), Ni(PPh3)(η5-C5H5) (9b), Au(PPh3) (10b)) and [M](C
C-4-C10H6-1-NO2) ([M] = trans-[RuCl(dppe)2] (6b), trans-[RuCl(dppm)2] (7b), Ru(PPh3)2(η5-C5H5) (8b), Ni(PPh3)(η5-C5H5) (9b), Au(PPh3) (10b)) and [M](C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-10-C14H8-9-NO2) ([M] = trans-[RuCl(dppe)2] (6c), trans-[RuCl(dppm)2] (7c), Ru(PPh3)2(η5-C5H5) (8c), Ni(PPh3)(η5-C5H5) (9c), Au(PPh3) (10c)) were synthesized and their identities were confirmed by single-crystal X-ray diffraction studies. Electrochemical studies and a comparison to the 1-nitrophenyl-4-alkynyl analogues [M](C
C-10-C14H8-9-NO2) ([M] = trans-[RuCl(dppe)2] (6c), trans-[RuCl(dppm)2] (7c), Ru(PPh3)2(η5-C5H5) (8c), Ni(PPh3)(η5-C5H5) (9c), Au(PPh3) (10c)) were synthesized and their identities were confirmed by single-crystal X-ray diffraction studies. Electrochemical studies and a comparison to the 1-nitrophenyl-4-alkynyl analogues [M](C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-4-C6H4-1-NO2) ([M] = trans-[RuCl(dppe)2] (6a), trans-[RuCl(dppm)2] (7a), Ru(PPh3)2(η5-C5H5) (8a), Ni(PPh3)(η5-C5H5) (9a), Au(PPh3) (10a)) reveal a decrease in oxidation potential for ruthenium and nickel complexes on proceeding from the phenyl- to naphthalenyl- and then anthracenyl-containing bridge. HOMO → LUMO transitions characteristic of MC
C-4-C6H4-1-NO2) ([M] = trans-[RuCl(dppe)2] (6a), trans-[RuCl(dppm)2] (7a), Ru(PPh3)2(η5-C5H5) (8a), Ni(PPh3)(η5-C5H5) (9a), Au(PPh3) (10a)) reveal a decrease in oxidation potential for ruthenium and nickel complexes on proceeding from the phenyl- to naphthalenyl- and then anthracenyl-containing bridge. HOMO → LUMO transitions characteristic of MC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-1-C6H4 to 4-C6H4-1-NO2 charge transfer red-shift and gain in intensity on proceeding to the ruthenium complexes; the low-energy transitions have increasing ILCT character on proceeding from the phenyl- to naphthalenyl- and then anthracenyl-containing bridge. Spectroelectrochemical studies of the Ru-containing complexes reveal the appearance of low-energy bands corresponding to chloro-to-RuIII charge transfer that red-shift on proceeding from the phenyl- to naphthalenyl- and then anthracenyl-containing bridge. Second-order nonlinear optical (NLO) studies at 1064 nm employing ns pulses and the hyper-Rayleigh scattering technique reveal an increase in quadratic optical nonlinearity upon introduction of metal to the precursor alkyne to afford alkynyl complexes and on proceeding from ligated-gold to -nickel and then to -ruthenium for a fixed alkynyl ligand. Quadratic NLO data of the gold complexes optically transparent at the second-harmonic wavelength reveal an increase in βHRS on proceeding from the phenyl- to the naphthalenyl-containing complex. Broad spectral range third-order nonlinear optical studies employing fs pulses and the Z-scan technique reveal an increase in two-photon absorption cross-section on replacing ligated-gold by -nickel and then -ruthenium for a fixed alkynyl ligand. Computational studies undertaken using time-dependent density functional theory have been employed to assign the nature of the key optical transitions and suggest that the significant optical nonlinearities observed for the ruthenium-containing complexes correlate with the low-energy formally Ru → NO2 band which possesses strong MLCT character, while the more moderate nonlinearities of the gold complexes correlate with a band higher in energy that is primarily ILCT in character.
C-1-C6H4 to 4-C6H4-1-NO2 charge transfer red-shift and gain in intensity on proceeding to the ruthenium complexes; the low-energy transitions have increasing ILCT character on proceeding from the phenyl- to naphthalenyl- and then anthracenyl-containing bridge. Spectroelectrochemical studies of the Ru-containing complexes reveal the appearance of low-energy bands corresponding to chloro-to-RuIII charge transfer that red-shift on proceeding from the phenyl- to naphthalenyl- and then anthracenyl-containing bridge. Second-order nonlinear optical (NLO) studies at 1064 nm employing ns pulses and the hyper-Rayleigh scattering technique reveal an increase in quadratic optical nonlinearity upon introduction of metal to the precursor alkyne to afford alkynyl complexes and on proceeding from ligated-gold to -nickel and then to -ruthenium for a fixed alkynyl ligand. Quadratic NLO data of the gold complexes optically transparent at the second-harmonic wavelength reveal an increase in βHRS on proceeding from the phenyl- to the naphthalenyl-containing complex. Broad spectral range third-order nonlinear optical studies employing fs pulses and the Z-scan technique reveal an increase in two-photon absorption cross-section on replacing ligated-gold by -nickel and then -ruthenium for a fixed alkynyl ligand. Computational studies undertaken using time-dependent density functional theory have been employed to assign the nature of the key optical transitions and suggest that the significant optical nonlinearities observed for the ruthenium-containing complexes correlate with the low-energy formally Ru → NO2 band which possesses strong MLCT character, while the more moderate nonlinearities of the gold complexes correlate with a band higher in energy that is primarily ILCT in character.


 Please wait while we load your content...
Please wait while we load your content...