A computational mechanistic study of Pd(ii)-catalyzed γ-C(sp3)–H olefination/cyclization of amines: the roles of bicarbonate and ligand effect†
Abstract
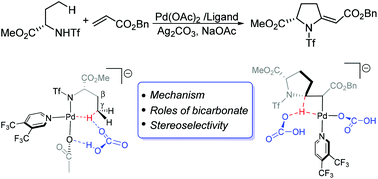
The detailed mechanism of palladium-catalyzed γ-C(sp3)–H olefination/cyclization of triflyl-protected amines was investigated by density functional theory (DFT) calculations. The olefinated intermediate was initially formed in the first catalytic cycle involving ligand exchange, bicarbonate-assisted C(sp3)–H bond cleavage, alkene insertion and ‘reductive β-hydride elimination’. The following syn-addition and reductive elimination furnish the aza-Wacker product. The first step of reductive elimination is the rate-determining step. The mechanism unveils the important roles of bicarbonate: aiding the C–H activation and abstracting the β-proton in the second step of reductive elimination. The parallel bridging mode in the metal-olefin intermediate facilitates the syn-addition, explaining the experimentally observed stereoselectivity. The effect of the monodentate pyridine-based ligands is also discussed.


 Please wait while we load your content...
Please wait while we load your content...