Formate complexes of titanium(iv) supported by a triamido-amine ligand†
Abstract
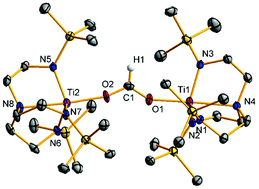
The terminal formate complex [(OCHO)Ti(N3N)] (3) containing the trianionic triamido-amine ligand (Me3SiNCH2CH2)3N3− (N3N) was prepared via salt metathesis of [ClTi(N3N)] (1) with sodium formate or alternatively by treatment of the alkyl complex [nBuTi(N3N)] (2) with ammonium formate [HNEt3][OCHO]. Deprotonation of 3 with potassium hexamethyldisilazide gave a polymeric helical chain of the oxo complex {K[OTi(N3N)]}n (4). Reaction of 2 with the trityl salt [Ph3C][B(3,5-Cl2C6H3)4] or the Brønsted acid [HNEt3][B(C6F5)4] gave [(Et2O)Ti(N3N)][BR4] (6[BR4]·Et2O) with R = 3,5-Cl2C6H3 or C6F5. The diethyl ether ligand was easily replaced by other L-type donor ligands such as tetrahydrofuran, pyridine, and 4-dimethylaminopyridine to give 6[BR4]·L with L = thf, py, and dmap. Reaction of 6[BR4]·Et2O with a stoichiometric amount of CO2 gave the dimeric, dicationic bis(carbamate)-bridged complexes [Ti{N(CH2CH2NSiMe3)2(CH2CH2NSiMe3(μ-CO2-ηO:ηO′))}]2[BR4]2 (7[BR4]2) through insertion of one CO2 into one of the titanium-amido bonds. Addition of pyridine to 7[B(C6F5)4]2 formed the monomeric carbamate complex [(py)Ti{((O2C-κ2O,O′)NSiMe3CH2CH2)N(CH2CH2NSiMe3)2}][B(C6F5)4] (8[B(C6F5)4]·py). The cationic formate-bridged species [(Ti(N3N))2(μ-OCHO-ηO:ηO′)][BR4] (10[BR4]) readily formed when the terminal formate complex 3 was reacted with the cationic 6[BR4]. The reactivity of triamido-amine stabilized titanium(IV) complexes is shown to differ considerably from that of related titanium tris(anilide) complexes.


 Please wait while we load your content...
Please wait while we load your content...