An intramolecular ortho-assisted activation of the silicon–hydrogen bond in arylsilanes: an experimental and theoretical study†
Abstract
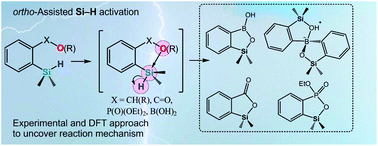
An intramolecular activation of the Si–H bond in arylsilanes by selected ortho-assisting functional groups based on boron, carbon and phosphorus was investigated experimentally and by means of theoretical calculations. The major conclusion drawn is that the presence of a negatively charged oxygen atom in the functional group is essential for providing effective chelation to the silicon atom which in turn results in the increased hydridic character of a resulting five-coordinated species. In contrast, an intermolecular attack of hydroxide on the silicon atom in aryldimethylsilane results in the activation of the silicon–aryl bond. This increased reactivity of the Si–H bond in intramolecularly coordinated arylsilanes can be ascribed to a significant trans effect which operates in the preferred configuration. Hydrolytic cleavage of the Si–H bond results in dihydrogen elimination and the formation of various silicon heterocyclic systems such as benzosiloxaboroles, spiro-bis(siloxa)borinate, benzosilalactone and benzophosphoxasilole. In addition, intermolecular reduction of benzaldehydes with ortho-boronated arylsilane was observed whereas compounds bearing other reducible functional groups (COMe, COOEt, CN and NO2) were inert under comparable conditions. Specifically, an intramolecular reduction of the CN group in an ortho-silylated benzonitrile derivative was observed. The mechanism of Si–H bond activation was investigated by the DFT theoretical calculations. The calculations showed that the intramolecular coordination of the silicon atom effectively prevents the cleavage of the Si–aryl bond. Furthermore, the reaction is favored in anionic systems bearing COO−, B(OH)3− or CH2O− groups, while in the case of neutral functional groups such as PO(OEt)2 the process is much slower.


 Please wait while we load your content...
Please wait while we load your content...