Mechanistic insights into the tropo-inversion of the biphenyl moiety in chiral bis-amido phosphites and in their palladium(ii) complexes†
Abstract
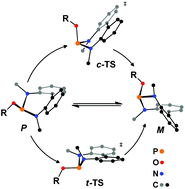
Chiral bis-amido phosphites L1 and L2 containing a diaminobiphenyl unit and a chiral alkoxy group derived from either (−)-menthol or 3-acetoxy deoxycholic methyl ester have been synthesised. Both L1 and L2 react with PdCl2(NCPh)2 affording di- or mononuclear derivatives with formula trans-[Pd(μ-Cl)Cl(L)]2 (1a, L = L1; 1b, L = L2) or trans-PdCl2(L)2 (2a, L = L1; 2b, L = L2) depending on the Pd : L molar ratio. The crystal structure of (M,P)-1a confirms the trans arrangement of the ligand L1 and shows an unusual puckering of the Pd2(μ-Cl)2 core (θ 46°). Both the ligands L1 and L2 and their complexes (1 and 2) are fluxional in solution as a consequence of the tropo-inversion of the diaminobiphenyl unit. For L1, L2, 1a and 2a a combined study including variable temperature 31P{1H} NMR spectroscopy and line shape analysis, Eyring plots and DFT calculations have shed light on the mechanism of the tropo-inversion.


 Please wait while we load your content...
Please wait while we load your content...