Boron-phil and boron-phob structure units in novel borides Ni3Zn2B and Ni2ZnB: experiment and first principles calculations†
Abstract
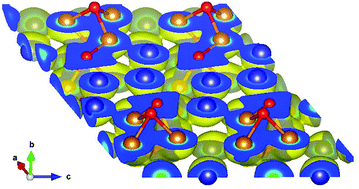
The crystal structures of two novel borides in the Ni–Zn–B system, τ5-Ni3Zn2B and τ6-Ni2ZnB, were determined by single crystal X-ray diffraction (XRSC) in combination with selected area electron diffraction in a transmission electron microscope (SAED-TEM) and electron probe microanalysis (EPMA). Both compounds crystallize in unique structure types (space group C2/m, a = 1.68942(8) nm, b = 0.26332(1) nm, c = 0.61904(3) nm, β = 111.164(2)°, RF = 0.0219 for Ni3Zn2B, and space group C2/m, a = 0.95296(7) nm, b = 0.28371(2) nm, c = 0.59989(1) nm, β = 93.009(4)°, RF = 0.0163 for Ni2ZnB). Both compounds have similar building blocks: two triangular prisms centered by boron atoms are arranged along the c-axis separated by Zn layers, which form empty octahedra connecting the boron centered polyhedra. Consistent with the (Ni+Zn)/B ratio, isolated boron atoms are found in τ5-Ni3Zn2B, while B–B pairs exist in τ6-Ni2ZnB. The crystal structure of Ni2ZnB is closely related to that of τ4-Ni3ZnB2, i.e. Ni2ZnB can be formed by removing the nearly planar nickel layer in Ni3ZnB2 and shifting the origin of the unit cell to the center of the B–B pair. The electrical resistivity and specific heat of τ5-Ni3Zn2B reveal the metallic behavior of this compound with an anomaly at low temperature, possibly arising from a Kondo-type interaction. Further analysis on the lattice contribution of the specific heat reveals similarity with τ4-Ni3ZnB2 with some indications of lattice softening in τ5-Ni3Zn2B, which could be related to the increasing metal content and the absence of B–B bonding in τ5-Ni3Zn2B. For the newly found phases, τ5-Ni3Zn2B and τ6-Ni2ZnB as well as for τ3-Ni21Zn2B20 and τ4-Ni3ZnB2 density functional theory (DFT) calculations were performed by means of the Vienna Ab initio Simulation Package (VASP). Total energies and forces were minimized in order to determine the fully relaxed structural parameters, which agree very well with experiment. Energies of formations in the range of −25.2 to −26.9 kJ mol−1 were calculated and bulk moduli in the range of 179.7 to 248.9 GPa were derived showing hardening by increasing the B concentration. Charge transfer is discussed in terms of Bader charges resulting in electronic transfer from Zn to the system and electronic charge gain by B. Ni charge contributions vary significantly with crystallographic position depending on B located in the neighbourhood. The electronic structure is presented in terms of densities of states, band structures and contour plots revealing Ni–B and Ni–Zn bonding features.


 Please wait while we load your content...
Please wait while we load your content...