Self-assembly of singlet-emitting double-helical silver dimers: the curious coordination chemistry and fluorescence of bisquinolylpyridone†
Abstract
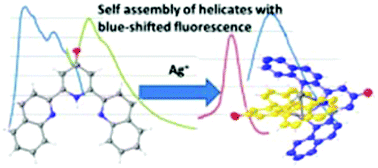
2,6-Bis(2-quinolyl)-4(1H)-pyridone 1, a novel quinoline analogue of the well-known ligand 2-terpyridone, shows unusual fluorescence with a large Stokes shift and low energy emission. Pyridine-pyridone tautomerism is investigated by NMR and theoretical methods and indicates that the low energy emission is from the pyridine form. 1 reacts with Ag(I) salts to give a double helical Ag2N6 core showing a BLUE shift in fluorescence with respect to the free ligand, which has been characterised experimentally and theoretically as involving an unusual mixed MLCT/ILCT excited state and emission from a singlet state.


 Please wait while we load your content...
Please wait while we load your content...