Evaluation of the metal-dependent cytotoxic behaviour of coordination compounds†
Abstract
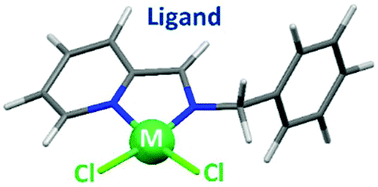
The [Cu(L)Cl2]2 and [Pt(L)Cl2] complexes were prepared from the simple Schiff-base ligand (E)-phenyl-N-((pyridin-2-yl)methylene)methanamine (L) and respectively, CuCl2 and cis-[PtCl2(DMSO)2]. DNA-interaction studies revealed that the copper complex most likely acts as a DNA cleaver whereas the platinum complex binds to the double helix. Remarkably, cell-viability experiments with HeLa, MCF7 and PC3 cells showed that [Cu(L)Cl2]2 is an efficient cytotoxic agent whereas [Pt(L)Cl2] is not toxic, illustrating the crucial role played by the nature of the metal ion in the corresponding biological activity.


 Please wait while we load your content...
Please wait while we load your content...