Boron calixphyrin complexes: exploring the coordination chemistry of a BODIPY/porphyrin hybrid†
Abstract
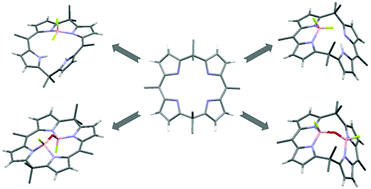
Boron complexes of calix[4]phyrins (1.1.1.1) were prepared by reacting the free-base ligands with BF3·Et2O. The reaction conditions can be efficiently tailored to produce mono- or di-boron calixphyrins. Mono-BF2 calixphyrins with boron coordinating to either the dipyrrin, BF2[H(Calix)], or dipyrromethane, BF2[H(Calix)] and BF2[H2(Calix)]+, bonding sites were isolated. The dipyrromethane isomer, BF2[H(Calix)], isomerises into BF2[H(Calix)] which kinetic studies and DFT calculations indicate is an intramolecular process. Two isomers of B2OF2(Calix) were isolated, one isomer bonding via the dipyrrin sites with the FBOBF moiety in cisoid geometry, and the second isomer bonding via the dipyrromethane sites with the FBOBF moiety in transoid geometry. Although the cisoid/dipyrrin isomer was calculated to be most energetically favourable for B2OF2(Calix), the isolation of the transoid/dipyrromethane isomer is postulated to occur via the presumed intermediate (BF2)2(Calix), for which DFT indicated a preference for transoid/dipyrromethane geometry.
- This article is part of the themed collection: Philip Power at 65: an icon of organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...