Displacement of η5-cyclopentadienyl ligands from half-sandwich C,C-(NHC-cyanoalkyl)–nickel(ii) metallacycles: further insight into the structure of the resulting Cp-free nickelacycles and a catalytic activity study†
Abstract
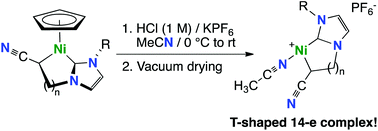
Four cationic C,C-(NHC-cyanoalkyl)–nickel(II) metallacyclic complexes, [Ni{Me-NHC-CH2CH(CN)}(NCMe)](PF6) (2a), [Ni{Mes-NHC-CH2CH(CN)}(NCMe)](PF6) (2b), [Ni{Mes-NHC-(CH2)2CH(CN)}(NCMe)](PF6) (2c) and [Ni{DiPP-NHC-(CH2)2CH(CN)}(NCMe)](PF6) (2d), were prepared by the removal of the Cp ligand under acidic conditions at 0 °C from the corresponding half-sandwich nickelacycles [NiCp{R-NHC-(CH2)nCH(CN)}] (1a–1d; Cp = η5-C5H5; n = 1 or 2; R-NHC-(CH2)nCH(CN) = 1-R-3-[(CH2)nCH(CN)]-imidazol-2-ylidene). Full characterization of 2a–d by 1H and 13C{1H} NMR spectroscopy in CD3CN and pyridine-d5, ATR-FTIR spectroscopy, mass spectrometry, and CHN microanalyses established the presence of only one acetonitrile ligand per nickel atom in the solid state. A DFT structural study conducted on the cations of the methyl-substituted 5-membered nickelacycle 2a and the mesityl-substituted 6-membered cycle 2c found a small energetic cost (ΔG = 7–12 kcal mol−1) for the loss of one acetonitrile ligand from the square-planar structures existing in solution, that should be easily amenable upon solvent evaporation (ΔG‡ = 14 kcal mol−1 in the case of 2c). Two structures with one acetonitrile ligand could be optimized in both cases: (i) a truly T-shaped 14-electron structure with an end-on acetonitrile ligand, and (ii) a masked T-shaped structure stabilized by the π-coordination of the dangling CN group of the metallated alkyl chain, the latter being favoured by 2.4 kcal mol−1 in the case of the flexible 6-membered ring 2c. A comparison of calculated vibrational frequencies with experimental FTIR spectra ruled out π-coordination of the dangling CN group as a ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) band at low frequency was absent. Complexes 2a–d thus probably exist as rare three-coordinate T-shaped 14-electron species in the solid state. Their catalytic activity was studied for the direct arylation of azoles, and 2c proved to be moderately active for the coupling of benzothiazole with aryl iodides. Mechanistic insights suggest that competing processes or a radical process catalysed by nickel particles could follow an initial reduction of 2c by the dimerization of a sacrificial amount of benzothiazole.
N) band at low frequency was absent. Complexes 2a–d thus probably exist as rare three-coordinate T-shaped 14-electron species in the solid state. Their catalytic activity was studied for the direct arylation of azoles, and 2c proved to be moderately active for the coupling of benzothiazole with aryl iodides. Mechanistic insights suggest that competing processes or a radical process catalysed by nickel particles could follow an initial reduction of 2c by the dimerization of a sacrificial amount of benzothiazole.


 Please wait while we load your content...
Please wait while we load your content...