A protic ionic liquid as an atom economical solution for palladium catalyzed asymmetric allylic alkylation†
Abstract
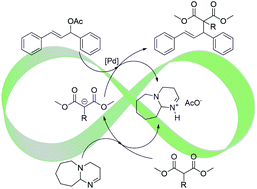
The asymmetric allylic alkylation of rac-1,3-diphenyl-3-acetoxyprop-1-ene (I) catalysed by palladium and diverse phosphorus containing ligands [(S)-BINAP, (R,R)-Chiraphite and (R,R)-Et-Duphos] in an ionic liquid [HDBU][OAc] was successfully performed, achieving full conversions and up to 96% ee of the (S)-product when (R,R)-Et-Duphos was used as a ligand. The reaction could be performed using an equimolar amount of substrate, malonate and base DBU, in which case the total products sum to the desired alkylated product and the ionic pair [HDBU][OAc]; this system thus produces its own IL solvent as the only co-product. These catalytic systems were active in recycling experiments for up to four cycles, albeit with a loss of activity due to the poor retention of palladium in the ionic liquid. The catalytic performance of each Pd/ligand system was optimized by varying the ratio of the substrate and malonate. Systems based on [HDBU][OAc] were found to be the best.


 Please wait while we load your content...
Please wait while we load your content...