The contradictory effect of the methoxy-substituent in palladium-catalyzed ethylene/methyl acrylate cooligomerization†
Abstract
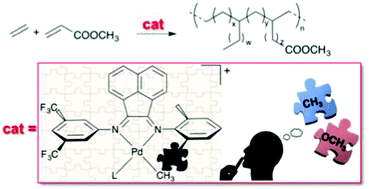
Two new nonsymmetric bis(aryl-imino)acenaphthene ligands (Ar,Ar′-BIAN) and one symmetric Ar2-BIAN were studied. The three ligands share the presence of at least one methoxy group on one of the two aryl rings. These ligands were used for the synthesis of neutral and monocationic palladium(II) complexes of general formula [Pd(CH3)Cl(N–N)] and [Pd(CH3)(L)(N–N)][PF6] (N–N = Ar,Ar′-BIAN, Ar2-BIAN; L = CH3CN, dmso). Due to the nonsymmetric nature of the ligands and their coordination to palladium in a nonsymmetric chemical environment, cis and trans isomers are possible for the three series of complexes with Ar,Ar′-BIANs. Both a detailed NMR investigation in solution and the X-ray characterization in the solid state point out that the trans isomer is the preferred species for the neutral derivatives, whereas for the cationic compounds a decrease in the stereoselectivity of the coordination is observed. One of the new Ar,Ar′-BIANs differs from an already reported nonsymmetric α-diimine for the replacement, on one aryl ring, of a methyl group with a methoxy substituent, thus allowing a comparison of the structural features of the relevant complexes. The monocationic complexes were tested as precatalysts for the ethylene/methyl acrylate copolymerization under mild reaction conditions. Despite the structural similarities observed in solution with the already known precatalysts, the present compounds demonstrated a remarkable decrease in the productivity values associated with a higher affinity for the polar monomer.


 Please wait while we load your content...
Please wait while we load your content...