Influence of the manganese and cobalt content on the electrochemical performance of P2-Na0.67MnxCo1−xO2 cathodes for sodium-ion batteries†
Abstract
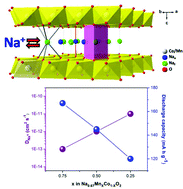
The resurgence of sodium-ion batteries in recent years is due to their potential ability to form intercalation compounds possessing a high specific capacity and energy density comparable to existing lithium systems. To comprehend the role of cobalt substitution in the structure and electrochemical performance of Na0.67MnO2, the solid solutions of P2-Na0.67MnxCo1−xO2 (x = 0.25, 0.5, 0.75) are synthesized and characterized. The XRD-Rietveld analysis revealed that the Co-substitution in Na0.67MnO2 decreases lattice parameters ‘a’ and ‘c’ resulting in the contraction of MO6 octahedra and the enlargement of inter-layer ‘d’ spacing. XPS indicates that the isovalent cobalt substitution in Na0.67MnO2 results in the partial/complete replacement of Jahn–Teller active trivalent manganese to form low-spin complexes of better structural stability. The Na-ion diffusion coefficient, DNa+, derived from cyclic voltammetry and impedance spectroscopy, confirmed the enhanced mass transport in Co-rich phases compared to Mn-rich phases. Furthermore, higher diffusion coefficient values are observed for Co3+/Co4+ than for their Mn3+/Mn4+ redox processes. In addition, Co-rich phases exhibit a high structural stability and superior capacity retention, whereas Mn-rich phases discharge higher capacities.


 Please wait while we load your content...
Please wait while we load your content...