Lysozyme and DNA binding affinity of Pd(ii) and Pt(ii) complexes bearing charged N,N-pyridylbenzimidazole bidentate ligands†
Abstract
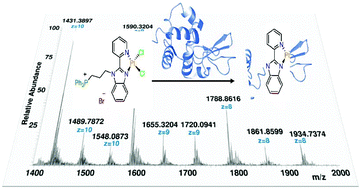
New cisplatin analogue Pd(II) and Pt(II) complexes bearing charged pyridylbenzimidazole derivatives furnished with either an alkylated sulfonate or a phosphonium side chain were synthesized, fully characterized and tested for their antimicrobial and cytotoxic activities against noncancerous human embryonic kidney cells (HEK293). The interactions with CT-DNA were investigated using UV/vis spectroscopy. Assignment of the electronic transitions was performed with the aid of TDDFT. The complexes showed interesting antifungal activity against Candida albicans and Cryptococcus neoformans. The Pd(II) complex conjugated with alkylated triphenylphosphonium exhibited higher cytotoxicity (CC50 = 8.932 μg mL−1, equivalent to 12 nM) than the others. The reactivity towards hen egg white lysozyme (HEWL) was investigated by electrospray ionization mass spectrometry. The formation of DNA- and HEWL-adducts was achieved via the noncovalent and covalent interactions.


 Please wait while we load your content...
Please wait while we load your content...