Isomerism and reactivity of nickel(ii) acetylacetonate bis(thiosemicarbazone) complexes†
Abstract
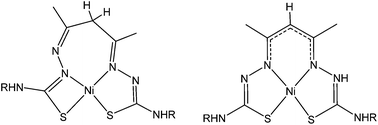
The complexation of nickel(II) with acetylacetonate bis(thiosemicarbazone) N2S2 ligands with varying substituents has revealed that two isomers can exist independently in solution. These isomers differ according to the formation of either a 5,6,5-membered (symmetric) or a 4,7,5-membered (asymmetric) chelate ring arrangement. These two isomers have distinctly different properties. The symmetric complex (sym-[Ni(acacR)]) is unstable in the presence of air and slowly converts to the oxidised analogue sym-[Ni(acacRO)] with a carbonyl group installed at the apical C-atom. The mechanism of this O-atom transfer reaction is still unclear but kinetic and spectroelectrochemical experiments in addition to Density Functional Theory calculations have identified a single electron oxidised NiII–ligand radical complex as a key intermediate. By contrast the asymmetric complex, asym-[Ni(acacR)] is inert to ligand oxidation.


 Please wait while we load your content...
Please wait while we load your content...