Ligand dynamics and protonation preferences of Rh and Ir complexes bearing an almost, but not quite, pendent base†
Abstract
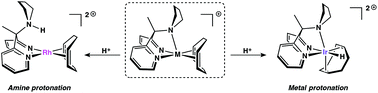
Pendent nucleophiles are essential partners in the cleavage and formation of bonds with hydrogen (e.g. protonation/deprotonation), but binding of the pendent group to the metal and the potential trapping of complexes in inactive states are a significant problem. The dipyridylmethane-based ligand framework bis(2-pyridyl)-N-pyrrolidinomethane (R,pyrCPy2), bearing a hemilabile pyrrolidine moiety, has been synthesized and complexes of the type [(R,pyrCPy2)M(COD)]X (COD = 1,5-cyclooctadiene) were prepared. The solution-phase ligand dynamics and relative protonation preferences were investigated via1H NMR spectroscopy; although favorable, pendent amine binding does not kinetically inhibit pendent base protonation. Protonation at the metal (with concomitant pyrrolidine binding) has been found to be favorable for Ir, whereas N-protonation is favorable for Rh. DFT calculations predict that the RhIII hydrides have much higher relative acidities than their Ir congeners (ΔpKa ≃ 7–8 in CH2Cl2), and are also more acidic than the strong acid [H(OEt2)2][B(C6F5)4].


 Please wait while we load your content...
Please wait while we load your content...