Diverse silver(i) coordination chemistry with cyclic selenourea ligands†
Abstract
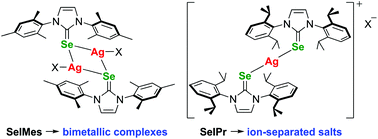
The coordination chemistry of two selenourea ligands (SeIMes and SeIPr) towards silver(I) triflate and silver(I) nitrate was investigated. Two aggregation modes were observed in the solid state, strongly influenced by the size of the aromatic substituents on the ligand. With mesityl groups, selenium-bridged bimetallic motifs [AgX(SeIMes)]2 were obtained, while for the bulkier diisopropylphenyl groups ion-separated species of formulae [Ag(SeIPr)2]+[X]− were obtained. Recrystallization of [Ag(NO3)(SeIMes)]2 from hot methanol resulted in the formation of a unique coordination polymer featuring three silver environments. Characterization of the complexes by NMR spectroscopy and mass spectrometry suggested all complexes adopt the ionic aggregation mode in methanol solution.


 Please wait while we load your content...
Please wait while we load your content...