Water oxidation by simple manganese salts in the presence of cerium(iv) ammonium nitrate: towards a complete picture†
Abstract
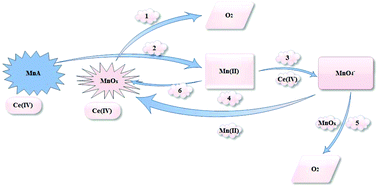
For the first time, using scanning electron microscopy, transmission electron microscopy, X-ray absorption near edge structure and extended X-ray absorption fine structure X-ray diffraction, it is showed that MnCO3, MnWO4, Mn3(PO4)2·3H2O, MnS and Mn(VO3)2·xH2O under the water-oxidation conditions and in the presence of cerium(IV) ammonium nitrate, are converted to Mn oxide without a high-range order. A mechanism is proposed for such conversion and as Mn oxide is an efficient water-oxidizing catalyst, it is thus a candidate as a contributor to the observed catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...