Ferrocenyl-sulfonium ionic liquids – synthesis, characterization and electrochemistry†
Abstract
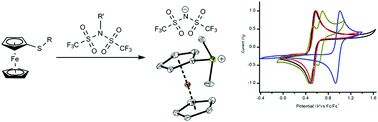
New ferrocenylsulfonium cation based ionic liquids were prepared by direct alkylation of the corresponding ferrocenyl-based thioethers with N-alkylbis(trifluoromethanesulfonyl)imides (R′TFSI). This convenient direct access to organometallic sulfonium bis(trifluoromethanesulfonyl)imide (TFSI) salts without the need for ion exchange was chosen in order to obtain highly pure and reversibly redox active room temperature ILs in many cases. In other cases the anion cation interaction in the solid state was studied by XRD analyses. Moreover a diferrocenylmethylsulfonium tetrafluoroborate with two redox active centers was synthesized. The redox chemistry of these sulfonium salts was investigated via cyclic voltammetry. Furthermore, UV-Vis spectra and thermoanalytical data are discussed. The electron-withdrawing sulfonium group is directly bonded to the ferrocenyl unit, therefore this cationic group influences the potential of these ionic liquids in a more pronounced way than being anchored to the ferrocenyl unit via an organic spacer. With their low absorbance in the visible light and reversible, tunable redox potential, these room temperature ILs open perspectives as redox mediators in dye sensitized solar cells (DSSCs), as redox electrolytes in supercapacitors or as overcharge protection additives in batteries.


 Please wait while we load your content...
Please wait while we load your content...