Nickel(iii)-mediated oxidative cascades from a thiol-bearing nickel(ii) precursor to the nickel(iv) product†
Abstract
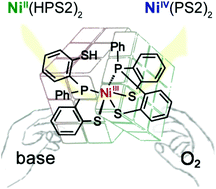
A nickel(II) complex, Ni(HPS2)2 (1) that contains two pendant thiols, is rapidly aerobically oxidized in the presence of an amine to produce a diamagnetic nickel(IV) complex, Ni(PS2)2 (2). This process was investigated spectroscopically at a temperature of −80 °C. Absorption spectra revealed that the deprotonation of one pendant thiol of 1 triggers an oxidative cascade; EPR findings indicate that single-spin species comprised of nickel(III) intermediates are produced in the reaction solution. Possible reaction routes were examined by DFT calculations, in which an energy profile indicates that (i) a self-driven formation of 2 favors a sequential proton/electron transfer pathway; (ii) kinetically trapped nickel(III) intermediates may respond to the specificity of the coordination of 2 in a cis-form. The overall findings help one to rationalize how a nickel(II) precursor can be oxidized by O2 to a higher oxidation state.


 Please wait while we load your content...
Please wait while we load your content...