Selective carbon dioxide sorption by a new breathing three-dimensional Zn-MOF with Lewis basic nitrogen-rich channels†
Abstract
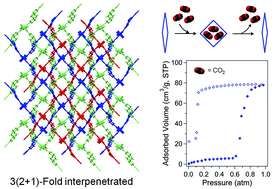
Lewis basic heteroatoms orderly located inside the well-defined channels of metal–organic frameworks (MOFs) are potentially ideal active sites for selective gas sorption and catalysis. To develop functional MOFs with Lewis basic sites inside channels, a new C2h-symmetric dicarboxylate-based bridging ligand, 3,3′-(pyrazine-2,5-diyl)dibenzoic acid (3,3′-PDBA), was prepared by a Suzuki coupling reaction. Subsequently, two new Zn-MOFs containing the C2h-symmetric 3,3′-PDBA bridging ligand and two different bis(pyridyl)-based pillars, 1,2-bis(4-pyridyl)ethane (bpa) or 1,2-bis(4-pyridyl)ethylene (bpe), were prepared through a thermal reaction in N,N-dimethylformamide (DMF). The resulting two Zn-MOFs of the general formula of three-dimensional (3D) [Zn2(μ4-3,3′-PDBA)2(μ2-bpa)]3·(DMF)5(H2O)13 (1) or 3D-like 2D [Zn2(μ4-3,3′-PDBA)2(μ2-bpe)]·(H2O) (2) displayed primitive cubic pcu net and 2D sql net, respectively. Both Zn-MOFs 1 and 2 contain uncoordinated Lewis basic pyrazinyl nitrogen atoms in the frameworks. The solvent-free 1 with flexible bpa linkers only showed a potential porosity of 15.9% by PLATON analysis. Zn-MOF 1 with openly accessible Lewis basic sites exhibited selective sorption of CO2 over N2, H2, and CH4 at low temperature. The adsorption and desorption isotherms for CO2 sorption at 196 K showed phenomenal hysteretic behaviour indicative of a breathing process through an adsorbate-discriminatory gate-opening process toward CO2 at a low gas pressure.


 Please wait while we load your content...
Please wait while we load your content...