Gallic acid-assisted synthesis of Pd uniformly anchored on porous N-rGO as efficient electrocatalyst for microbial fuel cells†
Abstract
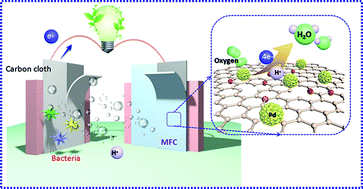
The sluggish kinetic rate-limiting oxygen reduction reaction (ORR) at the cathode remains the foremost issue hindering the commercialization of microbial fuel cells (MFCs). Utilization of the effect of micromolecule conjugation and the synergistic effect between Pd nanoparticles and N-rGO (nitrogen-doped reduced graphene oxide) to stabilize a precious metal onto carbon materials is a promising strategy to design and synthesize highly efficient cathode catalysts. In this study, gallic acid is used to facilitate the coupling of palladium (Pd) with N-rGO to form GN@Pd-GA via a simple hydrothermal process. Notably, the as-synthesized GN@Pd-GA as a cathode catalyst shows an approximately direct four-electron feature and demonstrates a high ORR performance in 0.1 M KOH. Furthermore, the stability and methanol tolerance of GN@Pd-GA are superior to those of the commercial Pt/C catalysts. In addition, a maximum power density of 391.06 ± 0.2 mW m−2 of MFCs equipped with GN@Pd-GA was obtained, which was 96.2% of the power density of MFCs equipped with a commercial Pt/C catalyst.


 Please wait while we load your content...
Please wait while we load your content...