An overview and recent progress in the chemistry of uranium extraction from seawater
Abstract
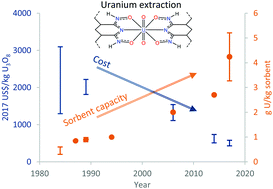
This review provides a brief background on the extraction of uranium from seawater as well as recent work by the United States Department of Energy on this project. The world's oceans contain uranium at 3 parts per billion, and despite this low concentration, there has been historical interest in harvesting it, mainly in Japan in the 1980s and the United States in this decade. Improvements in materials, chemistry, and deployment methods have all been made, with the ultimate goal of lower cost. This has been partially realized, dropping from approximately $2000 per kg U3O8 extracted in 1984 to $500 per kg today, although this is not yet competitive with terrestrial uranium. This technology may become cost-competitive if the cost of land-based uranium rises, especially if seawater extraction technology is improved further. The coordination chemistry aspects of the project are described in more detail, exploring the functional groups that are present on typical polymer sorbents as well as small-molecule analogues of these ligands. Selectivity for uranium over other metals, particularly vanadium, remains problematic, and techniques to both quantify binding strength and selectivity in order to overcome this issue are essential for future cost improvements.
- This article is part of the themed collection: 2018 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...