A new nitrogen rich open chain diazine ligand system: synthesis coordination chemistry and magneto-structural studies†
Abstract
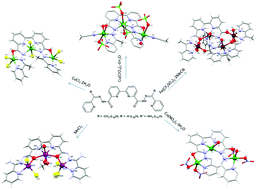
The synthesis and coordination chemistry of a new series of open chain diazine based ligands (L3aH2, L3bH2, and L3cH2) is reported. The ligands comprise a central disubstituted bipyridine moiety with two bridging alkoxide oxygen donors, together with diazine and pyridine terminal groups strategically located to coordinate three metal centres. Reactions of L3aH2 and L3bH2 with CuX2 (X− = ClO4, Cl, NO3) yield a trinuclear complex (1) and 1-D copper chains (2, 3). In these complexes the ligands bind copper ions via Nbipyridne, trans Ndiazine, Ohydrazone, and Npyridne donors while vacant sites are occupied by counter ions or solvent molecules (methanol, water, acetonitrile). Reaction of L3cH2 with MnCl2 affords a linear trinuclear Mn complex (4), where the Mn(II) ions are connected via μ2-Ohydrazone linkers with no N–N bridging. Reaction of L3aH2 with Fe(SO3CF3)2 yields a tetranuclear mixed valence Fe complex (5), in which both trans N–N and Ohydrazone bridging is observed. Magnetic studies reveal the presence of moderate to strong antiferromagnetic interactions in complexes 1–4 while a mix of ferromagnetic and antiferromagnetic interactions is observed in complex 5.


 Please wait while we load your content...
Please wait while we load your content...