The half Heusler system Ti1+xFe1.33−xSb–TiCoSb with Sb/Sn substitution: phase relations, crystal structures and thermoelectric properties
Abstract
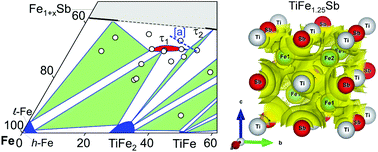
Investigations of phase relations in the ternary system Ti–Fe–Sb show that the single-phase region of the Heusler phase is significantly shifted from stoichiometric TiFeSb (reported previously in the literature) to the Fe-rich composition TiFe1.33Sb. This compound also exhibits Fe/Ti substitution according to Ti1+xFe1.33−xSb (−0.17 ≤ x ≤ 0.25 at 800 °C). Its stability, crystal symmetry and site preference were established by using X-ray powder techniques and were backed by DFT calculations. The ab initio modeling revealed TiFe1.375Sb to be the most stable composition and established the mechanisms behind Fe/Ti substitution for the region Ti1+xFe1.33−xSb, and of the Fe/Co substitution within the isopleth TiFe1.33Sb–TiCoSb. The calculated residual resistivity of Ti1+xFe1.33−xSb, as well as of the isopleths TiFe1.33Sb–TiCoSb, TiFe0.665Co0.5Sb–TiCoSb0.75Sn0.25 and TiFe0.33Co0.75Sb–TiCoSb0.75Sn0.25, are in a good correlation with the experimental data. From magnetic measurements and 57Fe Mössbauer spectrometry, a paramagnetic behavior down to 4.2 K was observed for TiFe1.33Sb, with a paramagnetic Curie–Weiss temperature of −8 K and an effective moment of 1.11μB per Fe. Thermoelectric (TE) properties were obtained for the four isopleths Ti1+xFe1.33−xSb, TiFe1.33Sb–TiCoSb, TiFe0.665Co0.5Sb–TiCoSb0.75Sn0.25 and TiFe0.29Co0.78Sb–TiCoSb0.75Sn0.25 by measurements of electrical resistivity (ρ), Seebeck coefficient (S) and thermal conductivity (λ) at temperatures from 300 K to 823 K allowing the calculation of the dimensionless figure of merit (ZT). Although p-type Ti1+xFe1.33−xSb indicates a semi-conducting behavior for the Fe rich composition (x = −0.133), the conductivity changes to a metallic type with increasing Ti content. The highest ZT = 0.3 at 800 K was found for the composition TiFe1.33Sb. The TE performance also increases with Fe/Co substitution and reaches ZT = 0.42 for TiCo0.5Fe0.665Sb. No further increase of the TE performance was observed for the Sb/Sn substituted compounds within the sections TiFe0.665Co0.5Sb–TiCoSb0.75Sn0.25 and TiFe0.33Co0.75Sb–TiCoSb0.75Sn0.25. However, ZT-values could be enhanced by about 12% via the optimization of the preparation route (ball-mill conditions and heat treatments).


 Please wait while we load your content...
Please wait while we load your content...