Induced chirality of cage metal complexes switched by their supramolecular and covalent binding†
Abstract
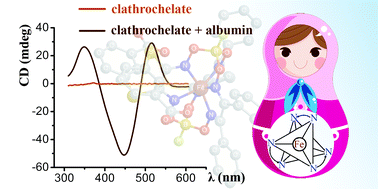
An ability of the ribbed-functionalized iron(II) clathrochelates to induce a CD output in interactions with a protein, covalent bonding or supramolecular interactions with a low-molecular-weight chiral inductor, was discovered. The interactions of CD inactive, carboxyl-terminated iron(II) clathrochelates with serum albumin induced their molecular asymmetry, causing an appearance of strong CD signals in the range of 350–600 nm, whereas methyl ester and amide clathrochelate derivatives remained almost CD inactive. The CD spectra of carboxyl-terminated clathrochelates on supramolecular interactions or covalent bonding with (R)-(+)-1-phenylethylamine gave a substantially lower CD output than with albumin, affected by both the solvent polarity and the isomerism of clathrochelate's ribbed substituents. In supramolecular assemblies, the bands were most intensive for ortho-substituted carboxyl-terminated clathrochelates. The ortho- and meta-phenylethylamide cage complexes in tetrachloromethane inverted the signs of their CD bands compared with those in acetonitrile. It was suggested that the tris-dioximate metal clathrochelates possess a Russian doll-like molecular system. Because of the distorted TP–TAP geometry, their coordination polyhedron had no inversion centre and possessed an inherent chirality together with the equiprobability of its left(Λ)- and right(Δ)-handle twists. The selective fixation of one of these C3-distorted conformations resulted in the appearance of the CD signal in the range of their visible metal-to-ligand charge transfer bands. Calculations by DFT methods were used to illustrate the possible conformations of the macrobicyclic molecules, as well as the intramolecular interactions between the cage framework and optically active distal substituents responsible for the chirality induction of the metal-centred coordination polyhedra.


 Please wait while we load your content...
Please wait while we load your content...