Alkaline-earth metal phenylphosphonates and their intercalation chemistry
Abstract
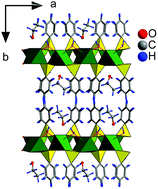
The intercalation chemistry of layered alkaline-earth metal phenylphosphonates with the general formula MeC6H5PO3·2H2O (Ca, Sr, Ba) is reviewed. The preparation of the host materials is described and their behavior in dependence on the relative humidity and pH of the reaction medium is discussed. Mutual relationships between MeC6H5PO3·2H2O and Me(C6H5PO3H)2 were investigated using a method of computer-controlled addition of reagents. The MeC6H5PO3·2H2O compounds are able to intercalate species having a free electron pair through the so-called coordination intercalation. In this way, 1-alkylamines, 1-alkanols, 1,n-diols and 1,2-diols were intercalated. In the case of the ethanol and methanol intercalates of strontium phenylphosphonate we were able to determine the structure of the host part by single-crystal X-ray diffraction. By combination of the data obtained from the diffraction with molecular modeling we suggested the arrangement of the host molecules in the interlayer space of the host. The arrangement of the shorter diols in the interlayer space of strontium phenylphosphonate was also proposed on the basis of molecular modeling calculations. These models help us to understand the structure of the prepared intercalates.
- This article is part of the themed collections: 2018 Frontier and Perspective articles and Recent Developments in Intercalation Compounds: Chemistry and Applications


 Please wait while we load your content...
Please wait while we load your content...