High-valent nitridorhenium(v) complexes containing PNP ligands: implications of ligand flexibility†
Abstract
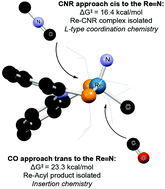
The synthesis of (PNP)Re(N)X (PNP = [2-P(CHMe2)2-4-MeC6H3]2N, X = Cl and Me) complexes is described. The methylnitridorhenium complex 3 was found to react differently with CO and isocyanides, leading to the isolation of a Re(V) acyl complex 4 and an isocyanide adduct 6. Two parallel pathways were observed for the reaction of 3 with CO: (1) CO inserts into the Re–Me bond to afford 4, and (2) 3 isomerizes by distortion of the aryl backbone of the PNP ligand to afford the isomer 3′. This is followed by the reaction of 3′ with CO to afford the tricarbonyl complex 5, which was fully characterized. The contrasting reaction of 3 with 2,6-dimethylphenyl isocyanide lends further support for the proposed isomerization pathway. DFT (M06) calculations suggest that insertion of CNR into the Re–Me bond (27.2 kcal mol−1) is inaccessible at room temperature. Instead the substrate adds to the metal center via the most accessible face i.e. syn to the rhenium–nitrido bond, to afford 6. The addition of CO to isomer 3′ is proposed to proceed with a similar mechanism to 2,6-dimethylphenyl isocyanide.


 Please wait while we load your content...
Please wait while we load your content...