Synthesis and applications to catalysis of novel cyclopentadienone iron tricarbonyl complexes†
Abstract
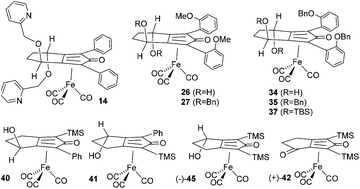
A series of cyclopentadienone iron tricarbonyl complexes with diverse structures were prepared, in each case using the intramolecular cyclisation of a diyne as a key step. The complexes were generated as enantiomerically enriched through (i) asymmetric synthesis of a C2-symmetric diol following a reported protocol, (ii) resolution of enantiomerically-enriched diastereoisomers formed from a chiral alcohol and (iii) kinetic resolution of a racemic ketone-containing iron tricarbonyl complex. The approaches underline the diversity of the synthetic routes which can be employed in the synthesis of homochiral cyclopentadienone iron tricarbonyl complexes. Although the complexes proved to be effective as catalysts for the reduction of ketones, the alcohol products were formed in low ees (not exceeding ca. 35%), highlighting the challenging nature of asymmetric catalysis using complexes of this type.


 Please wait while we load your content...
Please wait while we load your content...