Tuning the interlayer cations of birnessite-type MnO2 to enhance its oxidation ability for gaseous benzene with water resistance†
Abstract
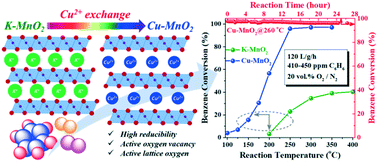
Benzene is a commonly-found air pollutant, which poses a great threat to human health and environmental development around the world. A layered birnessite-type manganese dioxide (MnO2) with a nanosheet morphology was synthesized by a simple and facile solution reaction in this study, which was further modified by Ce3+ and Cu2+ exchanges. For the pristine MnO2, benzene was hardly decomposed below 200 °C and only ∼40% conversion was achieved at a high temperature of 400 °C. However, Ce3+ and Cu2+ modifications significantly improved the activity of MnO2 for benzene decomposition. The Ce–MnO2 and Cu–MnO2 samples started to decompose benzene around 100 °C and nearly all benzene could be removed around 250 °C for ∼410 ppm benzene in dry gas under 120 L g−1 h−1 space velocity. Moreover, the Cu–MnO2 catalyst possessed the highest reaction rate (normalized by the surface area) among all the samples, and exhibited high resistance to high-temperature deactivation as well as good stability during continuous long-term testing. The origin of the tremendous effect of interlayer cations on the catalytic activity was studied via XRD, Raman, FT-IR, SEM, (HR)TEM, EDS mapping, BET, XPS and temperature-programmed techniques, which showed that the best performance of the Cu–MnO2 catalyst is attributed to it having the highest reducibility as well as highest lattice oxygen reactivity resulting from the larger number of active oxygen vacancies. Finally, the results of the humid stream reaction demonstrate that using Cu2+ exchange could significantly improve the water-resistant properties of MnO2 catalysts.


 Please wait while we load your content...
Please wait while we load your content...