Synthesis of a hierarchically porous niobium phosphate monolith by a sol–gel method for fructose dehydration to 5-hydroxymethylfurfural
Abstract
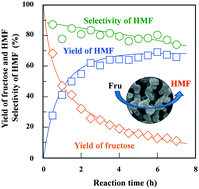
Hierarchically porous niobium phosphate (NbP) was synthesised via a sol–gel method accompanied by phase separation and employed as a catalyst for fructose dehydration. The niobium precursor was prepared by digesting ammonium niobate(V) oxalate in aqueous H2O2 with citric acid as a chelating agent to prevent the precipitation of niobium hydroxide. Poly(acrylamide) and poly(ethylene glycol) were used as phase separation inducers to produce co-continuous macropores and gel skeletons. The NbP sample calcined at 600 °C for 8 h had a high surface area (SBET ∼140 m2 g−1) and large acidity (0.84 mmol-NH3 g−1). The NbP monolith was amorphous after calcination at 600 °C and transformed into an α-NbOPO4 phase at 1000 °C. The NbP monolith calcined at 600 °C was an efficient acid catalyst for the dehydration of fructose to 5-hydroxymethylfurfural in water (70% yield) or in aqueous dimethyl sulfoxide (90% yield). The productivity of HMF reached 7.3 × 10−2 mol h−1 per kg-solution at an initial fructose concentration of 10.0 wt% in water with a catalyst loading of 2.0 wt%.


 Please wait while we load your content...
Please wait while we load your content...