Z-Selective alkyne semi-hydrogenation catalysed by piano-stool N-heterocyclic carbene iron complexes†
Abstract
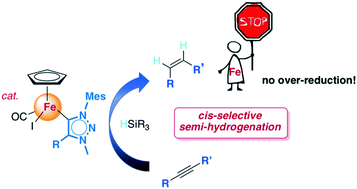
NHC iron(II) piano-stool complexes catalyse the selective semi-hydrogenation of alkynes to alkenes using silanes as reducing agents. Aromatic terminal alkynes are converted to styrenes without over-reduction to ethylbenzene derivatives. Furthermore, internal aryl alkynes afford cis-alkenes with excellent Z-selectivity.


 Please wait while we load your content...
Please wait while we load your content...