One-pot tandem conversion of monosaccharides and disaccharides to 2,5-diformylfuran using a Ru nanoparticle-supported H-beta catalyst†
Abstract
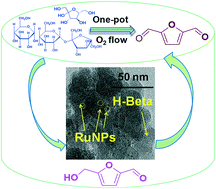
The direct, one-pot catalytic conversion of carbohydrates (especially sucrose and glucose) to 2,5-diformylfuran is a challenging task. The development of a bi-functional sustainable heterogeneous catalyst is crucial to achieve high selectivity for the desired product 2,5-diformylfuran in this direct conversion process. In this study, tandem, one-pot, two-step direct conversion of carbohydrates into 2,5-diformylfuran is reported using a Ru nanoparticle-supported H-beta catalyst. This carbohydrate conversion process is characterized by the abundance and low cost of reactants with no need for 5-hydroxymethylfurfural separation or purification. Molecular oxygen (1 atm) is used as an oxidant. No external oxidizing reagent is required to carry out this transformation. A combination of H-beta and Ru nanoparticles successfully affords the direct synthesis of 2,5-diformylfuran from carbohydrates via isomerization, dehydration, and successive selective oxidation under one-pot conditions. This one-pot tandem reaction protocol affords 2,5-diformylfuran with yields of 80% and 67% from fructose and sucrose, respectively. Moreover, the catalyst is easily recycled and reused without any loss of catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...