FeOx/Al2O3 catalysts for high-temperature decomposition of N2O under conditions of NH3 oxidation in nitric acid production†
Abstract
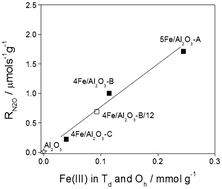
The catalytic activity of a series of FeOx/Al2O3 prepared under various conditions was evaluated for high temperature decomposition of N2O (HT-deN2O) in a complex gas mixture produced by oxidation of ammonia. Thus the relevant step in the industrial nitric acid production process was simulated. The catalyst stability during long-term exposure (12 days) to reaction conditions relevant to HT-deN2O was studied. Both fresh and aged FeOx/Al2O3 exhibited more than 90% conversion of N2O in the 750–900 °C temperature range. Structural analysis showed that the prepared FeOx/Al2O3 catalysts contained various proportions of δ, θ and α alumina phases and up to four individual iron species. In the δ and θ Al2O3 phases, well stabilized Fe(III) in Td or Oh coordination was identified as the active species in HT-deN2O.


 Please wait while we load your content...
Please wait while we load your content...