Melamine-derived graphitic carbon nitride as a new effective metal-free catalyst for Knoevenagel condensation of benzaldehyde with ethylcyanoacetate†
Abstract
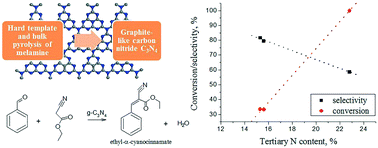
Graphitic carbon nitride was prepared by bulk and hard template pyrolysis of melamine. The prepared g-C3N4 as well as N-doped carbon used for comparison exhibited a reasonable catalytic activity in the Knoevenagel condensation between benzaldehyde and ethylcyanoacetate yielding ethyl-α-cyanocinnamate (conversion of benzaldehyde up to 100%, yield of the desired product up to 51%). Under the studied reaction conditions, weak basic sites assigned as C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C species are responsible for selective formation of the desired product. An increase of tertiary nitrogen content leads to an increase of benzaldehyde conversion with a simultaneous decrease of selectivity towards ethyl-α-cyanocinnamate due to a higher basicity. Carbon nitride obtained using silica as a hard template allows achieving the highest yield of the desired product (50.9%).
C species are responsible for selective formation of the desired product. An increase of tertiary nitrogen content leads to an increase of benzaldehyde conversion with a simultaneous decrease of selectivity towards ethyl-α-cyanocinnamate due to a higher basicity. Carbon nitride obtained using silica as a hard template allows achieving the highest yield of the desired product (50.9%).


 Please wait while we load your content...
Please wait while we load your content...