Insights into the dioxygen activation and catalytic mechanism of the nickel-containing quercetinase†
Abstract
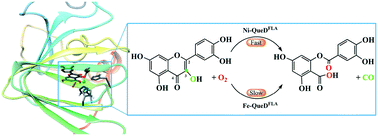
Quercetin 2,4-dioxygenase from Streptomyces sp. strain FLA (QueDFLA) is an enzyme of the monocupin family, which catalyzes the oxidative ring-cleaving reaction of quercetin using a nickel ion as the active site cofactor, and the iron ion that is necessary for most dioxygenases only shows low reactivity. To understand the reaction mechanism and the activation of dioxygen by the nickel ion, we performed QM/MM calculations to elucidate the reaction details and the special activation mechanism of this unique enzyme. Our calculations reveal two binding modes of dioxygen to the nickel ion, which can convert each other. Due to the overlap between the vacant d orbitals of nickel and the lone pair p orbitals of dioxygen and quercetin, electron transfer occurs from quercetin to dioxygen via the nickel center, thus, both dioxygen and quercetin can be activated by their binding to the nickel ion. On the basis of our calculations, the triplet reactant complex favors the catalytic reaction, and the whole reaction contains four elementary steps. In particular, a nonchemical process, the Op–Od bond rotation along the nickel center, is suggested to be rate-limiting with a free energy barrier of 19.9 kcal mol−1. NBO analysis reveals that it is the change of the coordination of Op with the nickel ion that leads to the high energy barrier of this process. In general, owing to the activation of the substrate and dioxygen by the nickel ion, the formation and collapse of the five-membered ring intermediate are quite easy, and the cleavage of O–O is in concert with the breaking of two C–C bonds. Furthermore, when the metal cofactor is replaced by an iron ion, the rate-limiting step switches from the Op–Od bond rotation to the collapse of the five-membered ring intermediate, corresponding to a free energy barrier of 30.3 kcal mol−1. This study sheds insight into the reaction mechanism of QueD and contributes to our general understanding of other nickel-containing enzymes.


 Please wait while we load your content...
Please wait while we load your content...